

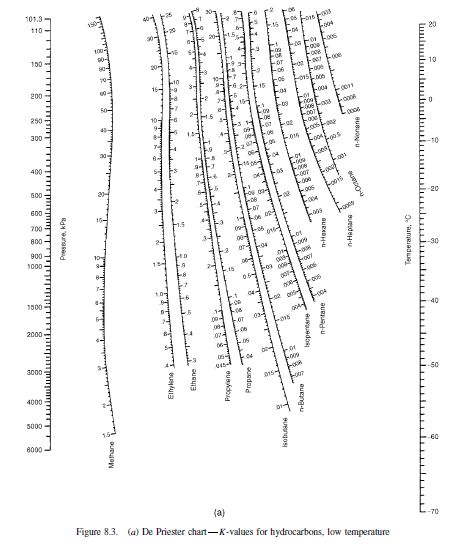
For light hydrocarbons, the value of Ki of each species can be obtained from the Verify K-Values of several Hydrocarbons It will now be convenient for us to work with K-Values in multicomponent systems High boiling point HC have LOW K-values Low boiling point HC have HIGH K-values The larger (heavier) the HC, the greater its BP, i.e. They will have mostly van der waal forces, i.e. According to chemistry, the hydrocarbons’ boiling point depends on their size, as Recall that K-Value is a relationship between liquid and vapor phases: In this section, we will cover only multiple-alkane systems For a Ternary (3 species in equilibrium) System, then we get: We have been studying binary systems, that is two species K-Values for Hydrocarbon Systems (dePriester) There I designed and modeled several processes relating separation of isopentane/pentane mixtures, catalytic reactors and separation processes such as distillation columns, flash separation devices and transportation of tank-trucks of product.įlash Distillation in Chemical and Process Engineering (Part 3 of 3) I worked as a Process Design/Operation Engineer in INEOS Koln, mostly on the petrochemical area relating to naphtha treating. I majored in Chemical Engineering with a minor in Industrial Engineering back in 2012. You will be able to continue with Batch Distillation, Fractional Distillation, Continuous Distillation and further courses such as Multi-Component Distillation, Reactive Distillation and Azeotropic Distillation. You will be able to understand mass transfer mechanism and processes behind Flash Distillation.
#Depriester chart propane software
Material and Energy Balances for flash systemsĪnimations and Software Simulation for Flash Distillation Systems (ASPEN PLUS/HYSYS)Īll theory is taught and backed with exercises, solved problems, and proposed problems for homework/individual study.
REVIEW: Of Mass Transfer Basics (Equilibrium VLE Diagrams, Volatility, Raoult's Law, Azeotropes, etc.)ĭistillation Theory - Concepts and PrinciplesĪpplication of Distillation in the IndustryĮquipment for Flashing Systems such as Flash Drums Understanding the concept behind Gas-Gas, Liquid-Liquid and the Gas-Liquid mass transfer interaction will allow you to understand and model Distillation Columns, Flashes, Batch Distillator, Tray Columns and Packed column, etc. MW_ethane = 30.07, MW_pentane = 72.15 Liquid densitites: rho_E = 0.54 g/ml (estimated), rho_p = 0.Binary Distillation is one of the most important Mass Transfer Operations used extensively in the Chemical industry. Assume the vapor is an ideal gas to calculate vapor densities. Find the dimensions in metric units required for a vertical flash drum. Find V/F, V, L, liquid mole fraction, vapor mole fraction. The drum operates p_drum = 700 kPa and T_drum = 30^degree C. We wish to flash distill a feed that is 55 mol% ethane and 45 mol% n-pentane. Find the mole fraction of n-butane, Z_B, in the feed. The feed mole fraction of ethane is z_E = 0.20. A flash drum operating at a pressure of 2.0 bar and a temperature of 0^degree C is separating a mixture of ethane (E), n-butane (B), and n-pentane (P). We wish a 90% recovery of n-hexane in the liquid (that is, 90% of the n-hexane in the feed exits in the liquid product). A flash drum operating at 300 kPa is separating a mixture that is fed in as 40 mol % isobutane, 25% n-pentane, and 35% n-hexane. Continue until your answer is within 0.5^degree C of the correct answer.

We desire a liquid that is 85 mol% n-hexane. Feed rate is 10 k mol/h, and drum pressure is 200 kPa. We wish to flash distill a feed that is 10 mol% propane, 30 mol% n-butane, and 60 mol % n-hexane.


 0 kommentar(er)
0 kommentar(er)
